Learn about the origins of the medical affairs function, how far it’s come and where it may be heading in the future as key proprietor of patient centricity
Words by Kirstie Turner
Once upon a time, there was an industry cursed with a negative reputation and a perceived lack of scientific integrity. Pharma’s fairy godmother appeared in the form of a much-needed department: medical affairs.
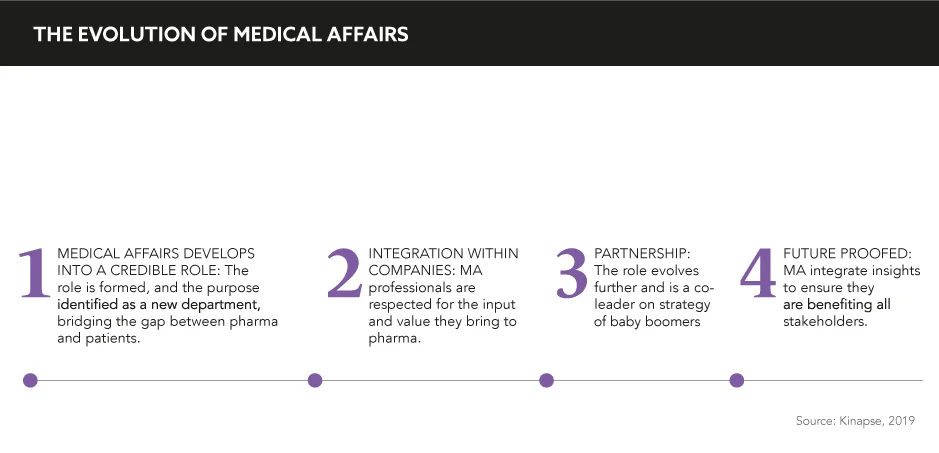
As the initial seeds of MA were sewn in the industry, no one could foretell just how integral its role would become. After years of nurturing, pruning, and nourishment, MA has truly blossomed, but what caused these changes and how will it continue to evolve?
Origin story
Michal Konštacký, Global Medical Affairs Director, Shire, reminds us what the pharma industry was like before MA: “I remember meeting a marketing director from a big pharma company at a congress in Belgium. I asked him where his medical counterpart was, and he replied that medics in pharma companies should stay at home and read articles.” Attitudes such as this led to a perception that pharma lacked scientific integrity: catastrophic for an industry at the forefront of scientific developments.
No tale is complete without conflict, and, with increasing negative publicity surrounding the industry, pharma were at risk of becoming the villain in their own story, as Ian Greenway, Medical Affairs Director, Complete HealthVizion, McCann Health Medical Communications, explains: “There was a trend towards negative publicity regarding the pharma industry and their influence over HCPs being linked to payments for conference attendance, entertaining, and gifts.”
The industry was forced to reconsider the structure of commercial and medical organisations
Current state of affairs
Fast forward to 2019 and “the situation has dramatically changed. This is mostly visible in biotech and rare diseases where the interface between the company is on the side of MA. This requires medics with different personalities, who are more proactive, strategic, and better educated as the interactions are more scientific than commercial”, says Konštacký.
But what has caused this evolution? “With an increasingly strict regulatory environment and a number of companies undergoing intense scrutiny over disguised promotion and unsubstantiated claims, the industry was forced to reconsider the structure of commercial and medical organisations. This led to the creation of separate MA organisations who had a clear reporting line through to medical leadership, rather than being linked to the commercial organisation”, says Greenway.
The advent of MA organisations signals an important chapter in the story of pharma, moving away from being purely commercial to recognising the importance of medical integrity.
Removing this heavily commercial influence was key, as Greenway continues: “The industry realised that it was essential to ensure a transparent relationship between their medically trained experts and HCPs so that medical communication and education regarding their clinical studies and products was conducted on a peer-to-peer basis, without commercial influence.”
Looking to the future
While there is no crystal ball to foresee what will become of MA in years to come, it is sure to remain an integral part of the industry, and indeed flourish further.
Greenway believes that: “The MA role will become further integrated with the value demonstration function as pressure to demonstrate a medicine’s value is increasingly important from the start of the development process. This requires early development of clear insight into future stakeholder needs, so that the medical strategy is developed to ‘build in’ these requirements from the early Phase I/II studies, rather than just considering these in Phase III trial design.” Increased importance is being placed on medical strategy: an element of pharma that could once have been their downfall.
Going forward, MA must continue to collaborate with field medical professionals to provide insight and allow development of knowledge into the research and development process. With increasing regulation comes more restricted access to HCPs for pharma’s sales departments and this channel of access must come from MA. Greenway continues: “MA will be the function that HCPs see as the face of the pharma company, which positions them in a unique position to transform the healthcare environment.”
As we enter the age of the patient, with personalised treatments and patient centricity at the forefront of pharma, these elements must play into MA’s plans for the future, as Greenway explains: “The advent of personalised medicine and tailored care packages for patients is transforming treatment algorithms and including a wider range of HCPs and the MA function should be ensuring that this transformation is built into their strategic medical plan early in the development phase.”
While MA’s function may grow and adapt even further in the coming years, the integral nature of the role is sure to remain constant, as Konštacký says: “I see MA as storytellers who help others to understand science with accuracy of their claims and help companies to maintain their scientific integrity.” As MA continues to blossom and create fruitful return for the industry that has nurtured it, the department is in prime position to help pharma live happily ever after.