The year 2022 was one of innovation in neurological treatment, but along with new therapies come access challenges. What can pharma do to ensure breakthrough treatments reach those in need?
Words by Jade Williams
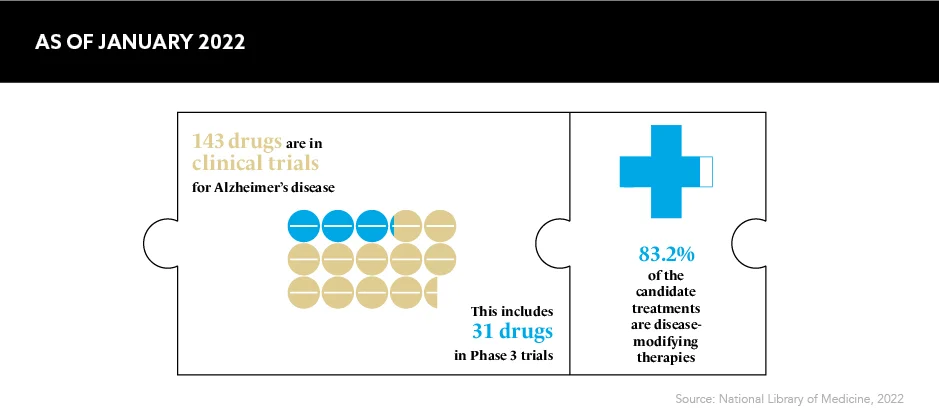
A whirlwind of successful discoveries hit neurology in 2022, offering patients a whole host of therapeutic advancements and enhanced treatment options. One of the most notable was the world’s first drug to slow Alzheimer’s disease reaching its primary endpoints in a Phase 3 trial in September 2022, which the charity Alzheimer’s Research UK commented was the “news we’ve all been waiting for”.
There is, however, concern arising from charities and the world’s leading healthcare providers. Their main issue? How the pharmaceutical industry can be sure all patients will be able to access and benefit from breakthrough treatments when these can be hugely complex for healthcare systems to deliver.
The timing’s right
The apparent acceleration in neurological treatments is far from a sudden phenomenon. The leaps and strides seen in neurology over the course of 2022 are the culmination of years of behind-the-scenes efforts from pharma companies and others.
The Alzheimer’s drug lecanemab, which is the brainchild of Eisai and Biogen, is one such breakthrough hoping to make a difference for patients. It is a treatment targeting amyloid proteins in the brain, and it won approval by FDA in January 2023. Speaking about the significance of this milestone on patients and their loved ones, Dr Billy Dunn, Director, Office of Neuroscience, FDA Center for Drug Evaluation and Research, says: “This treatment option is the latest therapy to target and affect the underlying disease process of Alzheimer’s, instead of only treating the symptoms of the disease.”
Focusing on the early stages of the disease and not just on the treatment of symptoms is a game changer for patients, and this results, in part, from an “improved mechanistic understanding of disease and improved trial design and tools”, according to Christine Eksteen, EMEA Medical Director, Eisai. These have “led to [a] renewed enthusiasm to invest in neurodegeneration research and drug development”, she says. What’s more, “increasing life expectancy has produced a dramatic rise in the prevalence and impact of ageing-associated diseases such as Alzheimer’s disease”, Eksteen adds, which has driven the industry’s motivation even more.
According to the World Health Organization, Alzheimer’s affects around 55 million people worldwide. This number is expected to reach 78 million by 2030 and 139 million by 2050. The burden this poses on health and social care systems, as well as on individual patients and their caregivers “has led to global leaders having set a deadline of 2025 for finding an effective way to treat or prevent Alzheimer’s disease”, says Eksteen.
The 2025 deadline is rapidly approaching. If the pharma industry hopes to stamp out the fast-growing fires of ageing-associated diseases, innovative treatments need to be discovered and approved alongside plans to distribute these to as many patients as possible.
Diagnostic downfalls
Effective delivery, however, may hang in the balance. In the US, FDA may have approved the drug, but the Centers for Medicare and Medicaid Services (CMS) and other insurance providers have denied coverage. Therefore, lecanemab and other treatments in its class may only be available to those who can afford to pay out-of-pocket.
“What the FDA did in granting accelerated approval to Leqembi [lecanemab] was the right decision. But what CMS is doing by severely restricting coverage for approved treatments is unprecedented and wrong,” says Joanne Pike, President and Chief Executive Officer, Alzheimer’s Association. “Without access to and coverage of this treatment and others in its class, people are losing days, weeks, months – memories, skills and independence. They’re losing time.”
Improved trial design and tools have led to the renewed enthusiasm to invest in neurodegeneration research
There have also been murmurs that healthcare systems could struggle to identify and treat eligible patients with these new treatments. NHS England cites a lack of infrastructure and staff to spot candidates, deliver the drug and perform the multiple MRI safety scans needed. The service simply lacks the resources to screen all at-risk patients and treat those at the mild cognitive impairment stage. As such, patients are often diagnosed and discharged.
An education gap in terms of Alzheimer’s causes and progression could be an influential factor in the diagnosis problem. Instating a culture of urgency by educating healthcare professionals on the importance of early diagnosis in diseases such as Alzheimer’s could ensure more patients are identified for treatment with innovative therapies. Getting these approved by regulatory bodies is only one step – education and screenings for diseases must both be prioritised, too.
Eksteen, however, does note that the industry has “highlighted the gaps and needs” that it will need to fill to get neurological treatments to patients, and, as such, has “sought to share international best practice and effective initiatives”.
In July 2022, the UK introduced integrated care boards responsible for developing plans to meet the health needs of their specific populations. Working collaboratively with these care boards could dramatically streamline patient care pathways and reduce diagnosis timelines – both of which are key priorities if patients are to benefit from breakthrough therapies such as lecanemab.
The road ahead
Although creating access to new neurological treatments is complex, the latest star paints an exciting picture of the future: a world eradicated of Alzheimer’s disease. While the intricacies of this can’t be accurately predicted, the creative minds in the field of neurology are paving the way for further clinical advancements.
FDA’s approval of lecanemab will soon be followed by a decision from the European Medicines Agency and, if approved, could send further waves throughout the industry. Whatever may lie in wait over the next few years, pharma must make certain that patients can access the treatments available to them. Partnering with healthcare to improve diagnostic timelines is simply the first step on a long road, which pharma should set out on with haste.