
Access to surgical abortions in the US is rapidly declining following the overturning of Roe v. Wade, and now medical abortions are under threat, too. How is access to the abortion pill mifepristone in jeopardy, and what is, or should, pharma be doing to help?
Words by Isabel O’Brien
On 24 June 2022, the US Supreme Court voted 5-4 to overturn the historic Roe v. Wade ruling. This decision has not only jeopardised access to safe and legal surgical abortions in the US but opened the floodgates for legislatures to close off avenues to medical abortions, too. Abortion drugs have a target on their back, and conservative critics are pulling back their bows.
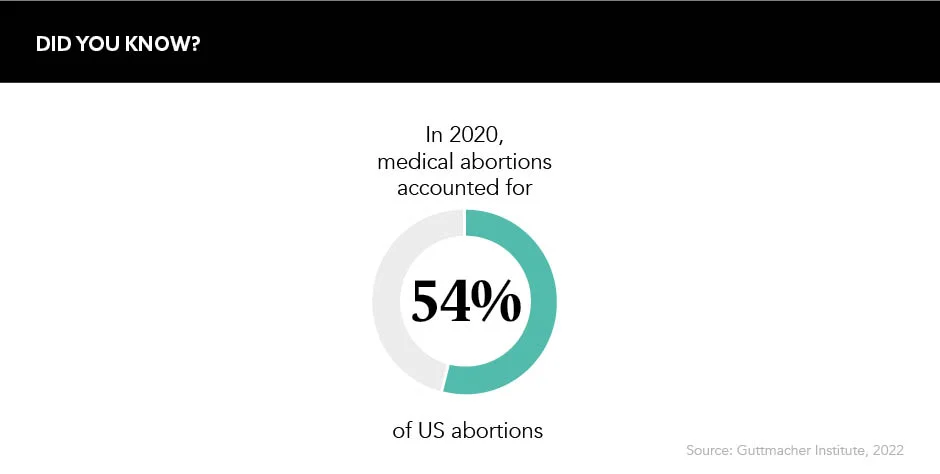
In 2020, over half of US abortions were medically induced, with the drug mifepristone, which is typically taken in combination with misoprostol, often being prescribed via telehealth and taken outside of a medical setting. The shuttering of abortion clinics has grabbed more media attention since the overturning of Roe v. Wade, but bids to cut off access to medical abortions could deal an even heavier blow.
Moving a safe and effective prescription birth control pill to OTC will help even more women and people access contraception without facing unnecessary barriers
Conservative campaigners are aggressively pursuing laws to prevent mifepristone from being prescribed under any circumstances and have been striving to make the drug difficult to obtain remotely. A total of 19 states have now banned prescription of the drug by telehealth, and Texas has just enacted a law that prohibits the distribution of abortion pills by post.
Speaking at the time of the ruling, US Attorney General Merrick Garland said that US states “may not ban mifepristone based on disagreement with the FDA’s expert judgment about its safety and efficacy”, but as more states seek to implement restrictions, clearly the risk of pregnant women not being able to easily access the drug is building.
How has pharma responded?
While many pharma companies have publicly objected to the overturning of Roe v. Wade, and sought to provide access to surgical abortion services for their employees, this issue of access to medical abortions is a highly convoluted beast in need of urgent attention.
Two companies under pressure to act are the manufactures of mifepristone for the US market: Danco Laboratories, which held the original patent, and GenBioPro, which won the right to produce a generic version in 2019. Advocates are calling for the firms to make the drug available without a prescription, or even to apply for a label extension that would approve its use during miscarriage so the drug would not be solely associated with abortion.
These are both sound ideas, in principle, but Danco Laboratories stated it will not be making any further applications to the FDA. A spokesperson told the Wall Street Journal: “The company is very moderately profitable, and the investors only have so much – it’s not a bottomless bucket for them.” In short, the company’s ability to act is limited by its size and lack of resource.
A transparent, open discussion among various healthcare experts is needed
On the other hand, GenBioPro has recently engaged a federal lobbying firm to advocate on its behalf on issues related to medication abortion, access to abortion services and matters tied to the FDA’s approval of mifepristone. A legal fight in Mississippi about in-person prescription laws is also underway. But still, this is a small firm up against a vast number of highly motivated Republicans, and its power to truly turn the tide on the current trajectory is slim.
Everyone’s problem
The issue of access to medical abortion is complex and isn’t going to be solved in a hurry. But as Samantha Miller, Co-Founder and Co-CEO, Cadence Health, notes, the wider pharma industry has the knowledge to try and find a solution. “The industry has expertise and perspective to contribute to the conversation,” she says. “A transparent, open discussion among various healthcare experts is needed.”
In the meantime, improving access to contraception is one way companies can play their part, as nearly one-third of adult US women who have tried to obtain a prescription or refill for contraceptive pill, patch or ring have reported difficulties in doing so. “Moving a safe and effective prescription birth control pill to OTC will help even more women and people access contraception without facing unnecessary barriers,” comments Frederique Welgryn, Chief Strategic Operations and Innovation Officer, HRA Pharma. The company is currently seeking FDA approval for what could be the country’s first ever non-prescription contraceptive – norgestrel, known as Opill.

In addition, organisations such as the Bill Gates Foundation are supporting the creation of refillable implants that could be worn longer and require fewer health centre visits. The foundation pledged $625,803 to a prototype being developed by chemical and specialty materials company Celanese in July 2022.
While the pharma industry is working to improve access to contraception and has condemned the ruling against Roe v. Wade, its action on the issue must not end here. Medical abortions are the most accessible, affordable and discreet terminations available. How can the industry defend abortion pills against the flying arrows of the anti-abortionists? Only time will tell.
A history of mifepristone in the US
The pill used for medical abortions, mifepristone has been contentious in the so-called land of liberty for some time. Developed by the French pharmaceutical company Roussel-Uclaf, it was approved in France in the late 1980s, but its journey to the US was protracted and troubled. Despite the drug being deemed “safe and effective” by the FDA in 1996, a bill amendment was passed in 1998 preventing the regulatory body from using taxpayers’ money to test, develop or approve any abortion drug. As a new century dawned, President Clinton managed to overturn the amendment, and the drug was approved by the FDA shortly after. Still, approval in the US came over a decade after those in Europe.





