Despite continued advances in personalised medicine, there is still more to be done to encourage physicians, policy makers and payers to back these life-changing solutions
Words by Saša Janković
From monogrammed designer bags and luxury stationery to your own bespoke coffee blends, there’s a whole host of things you can stamp your individuality on if you want to. And while all of these can zhuzh up your life, there’s another kind of personalisation that could just save it.
The intervention in question is personalised medicine – aka precision medicine – a fast-growing area of healthcare that combines information about an individual’s genome with other diagnostic and clinical data to predict and detect diseases, create specifically targeted treatments that minimise side effects and instigate appropriate preventative measures for future health risks.
It has been 25 years since the approval of Herceptin – commonly known as the ‘poster child’ for precision medicine – changed the outlook for many women diagnosed with HER2-positive breast cancer, which had previously been considered incurable. Now, precision or personalised medicines are rapidly entering the market and their transformative potential is being widely recognised.
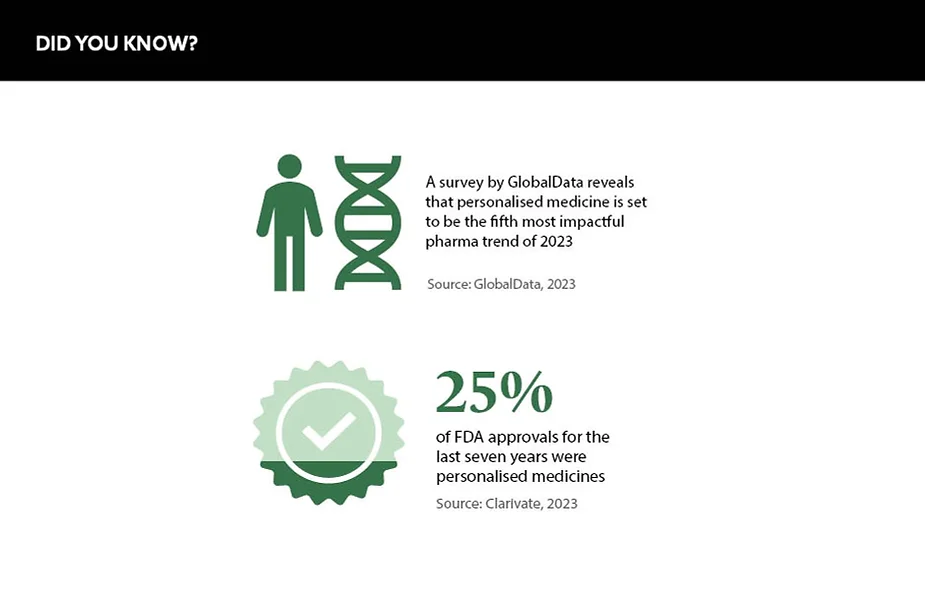
According to a Personalized Medicine Coalition (PMC) report ‘Personalized Medicine at FDA: The Scope & Significance of Progress in 2022’, more than one in four new FDA drug approvals in the past seven years have been for personalised medicines. In addition, GlobalData forecasts that personalised medicine will be the fifth most influential pharmaceutical trend in 2023.
Looking ahead
Combined with new technologies such as liquid biopsies and gene therapies, this trend suggests that personalised medicine is reshaping drug discovery and development – and there is more to it than meets the eye.
“Even though biotherapeutic development for cancer treatment and other genetic disorders is still at the forefront of personalised medicine, disruptive innovations in technology and data acquisition have led to the expansion of the field into other areas of disease and health management,” says Hakim Yadi, Co-Founder and CEO, Closed Loop Medicine, a techbio company developing prescription combination drug-plus-software products. He adds that, specifically, personalisation is being used to increase the effectiveness of existing drugs.
Investment remains a main hurdle
For example, one of the most critical elements of chronic disease management is the selection of a drug dose regimen that is both safe and effective for the individual. In the traditional healthcare model, most therapies are approved based on the overall safety and efficacy demonstrated in large-scale clinical trials, but in real life, the efficacy and tolerability of a given drug can vary dramatically from person to person. This can result in poorer outcomes for patients, higher healthcare costs for payers and lost revenue for pharma companies.
“Undertaking a thorough assessment of an individual’s response to medication through real-time feedback and using this information to dose optimise is key to improving patient outcomes,” says Yadi. “Therefore, integration of traditional therapeutics, medical devices and new technologies that enable real-time monitoring of patients are creating a huge opportunity to collect real-world data to help ensure personalised treatments are available for individual patients.”
Current challenges
Despite the continued existence of the Precision Medicine Initiative launched by President Barack Obama in 2015, the pharma industry continues to face challenges in convincing payers and healthcare agencies to rally behind precision medicine – including regulatory and reimbursement hurdles as well as a lack of infrastructure to identify eligible patients and ensure they have access to treatment.
Another hurdle to precision medicine is biomarkers. Joaquín Casariego García-Lubén, Group CEO, Aldebaran Health Intelligence Group, notes that utilising biomarkers in drug discovery is “still a long process, and investment remains a main hurdle”. Although, he adds that the Innovative Medicines Initiative and the European Commission’s big data programmes have been put in place to accelerate this.
Data science also presents a potential obstacle. According to Casariego García-Lubén, data science can be used to generate reliable historical control groups or synthetic control arms for single arm trials in low prevalence diseases, such as oncology, but he reveals that “authorities still show some reluctancy to endorse [these]”. Synthetic data holds enormous potential for the development of personalised therapies, but a change in attitudes is needed for its value to be widely harnessed in precision medicine.
Utilising biomarkers in drug discovery is still a long process
“Although natural language processing software can be applied to electronic medical records, accuracy and reliability are still work in progress,” adds Casariego García-Lubén. “Access to individual and networks of databases is also difficult considering data protection and privacy issues nowadays.” It’s not just attitudes that need to evolve, but also the data available to AI tools that work on personalised solutions.
What next?
A recent study by the PMC discovered only 34% of non-small cell lung cancer patients were getting the targeted therapies that would help them. Despite the many positive signals, continued progress in personalised medicine cannot be taken for granted.
This is a view held by Edward Abrahams, President, PMC, who says access shortfalls are partly due to “multiple practice gaps limiting the uptake of new [and] innovative medicines”. But what does this mean for medical affairs teams in terms of adapting their approach to engaging HCPs? Abrahams believes that MA teams should spend more time educating HCPs about the opportunities to improve care with personalised solutions.
In addition, the PMC’s ‘Personalized Medicine at FDA’ report argues that as scientists and innovators continue to develop groundbreaking personalised medicine tests and treatments, policymakers must continue to support policies that encourage the advancement of the field. The road to widely available personalised medicines is still a long one, but there is hope that it can be navigated with the right support.