Words by James Coker
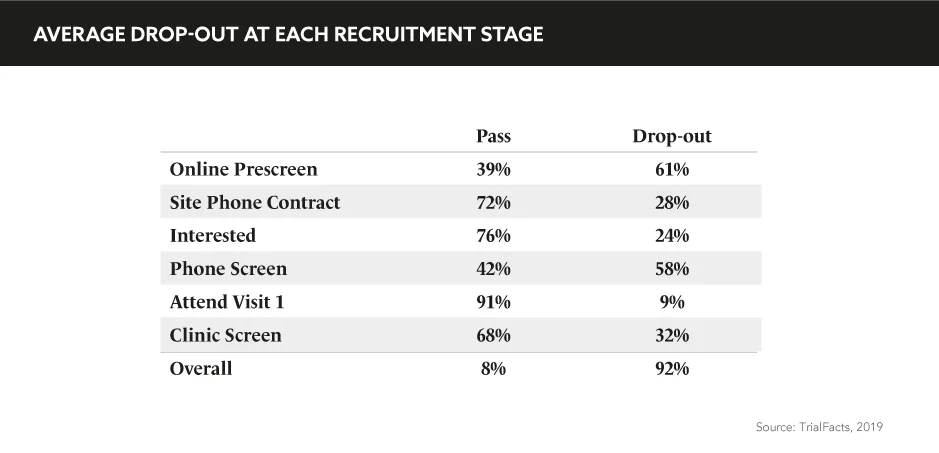
“Patient recruitment and retention remain the biggest challenges in clinical studies”, comments Gail Adinamis, CEO, GlobalCare Clinical Trials, during the eyeforpharma Patient Summit Europe. “It is not a sustainable model; we know that <5% of patients actually participate in studies and this is primarily because it’s not convenient or comfortable for them to do so. We also know that 20% of sites that are initiated never see a single patient, while 15–40% of patients are going to drop out before the end of the study.”
When concerns over a lack of geographic and ethnic diversity are mixed into Adinamis’ sobering analysis, it is surely time for the pharmaceutical industry to trial a new approach to clinical studies.
Patient recruitment and retention remain the biggest challenges in clinical studies
As always, diagnosis is the first step to finding an effective cure. In the traditional clinical trial model that remains prevalent throughout the life sciences sector, participants are obliged to travel back and forth from investigator sites to contribute. Such a model, unsurprisingly, presents difficulties for those with heavy work and family commitments. Notwithstanding the physical limitations that their condition may place upon them.
“If a patient is very far away, has a debilitating disease, doesn’t have transportation, and has family obligations, they may want as many home visits as possible”, says Adinamis. While patient-centric approaches are increasingly being woven into the fabric of pharma’s clothing, the set-up of clinical trials in R&D have, to date, remained steadfastly rigid, perhaps the equivalent of medieval chainmail. Adopting a flexible prose, centred around the needs of patients, must become a priority of R&D teams at the initiation of clinical studies.
Anna Cederberg, Co-Founder, Melio, considers the perverse quandary that while Justin Bieber can sell 40,000 seats for a concert twice in 30 seconds, a clinical trial of an Alzheimer’s treatment has yet failed to reach its target of 800 volunteers after 2 years. Just like any other service or product, in Cederberg’s view, user experience is fundamental to improving the recruitment issue. “This requires you to be creative and to have a product or a service that people actually want”, she says.
This is a principle Servier have adopted for a large, multinational, ongoing Phase III trial of an autism treatment for children and adolescents. To account for the varying needs of children and multiple caregivers, as well as cultural nuances at play with this condition, the traditional clinical trial paradigm was abandoned in favour of pragmatism.
“We decided to explore and build an environment around autistic children”, notes Marta Garcia, Director of Patient Service, R&D Clinical Development, Servier. She added: “We had to challenge our existing practice and explore resolutions to be flexible with each country, so we thought out of the box and learned by experience.”
Decentralised trials present a more general concept pharma can explore within clinical studies. “A decentralised trial includes studies at locations that are remote to the investigator site; it could be in patients’ homes, their workplace, or travel destination. And these are visits that are conducted through the use of mobile or local healthcare providers”, explains Adinamis.
These providers are able to remotely conduct services, such as blood draws and drug administration, in the presence of a qualified healthcare professional; several hundred have now been undertaken in recent years, to great effect. In a Phase I trial in which patients were administered a drug three-times a week, incredibly not a single participant dropped out. In another, in which homecare services were offered following study initiation, a mere 3% of those who used homecare services dropped out, compared with 67% of those who did not use this option.
A decentralised trial includes studies at locations that are remote to the investigator site
“Families, patients, and caregivers all appreciate the convenience and comfort of having these services done at a location and time that’s convenient for them”, says Adinamis. “Investigators are able to recruit patients from a much broader geographic area, have better compliance, and ensure geographic and ethnic diversity. And for sponsors, the development timeline is shorter; if they have faster recruitment and better retention, fewer patients have to be enrolled because there’s fewer dropouts. And most importantly, consumers get access to new therapies faster.”
In the words of Sir Winston Churchill, ‘to improve is to change; to be perfect is to change often’. In general terms, pharma has, to date, failed to amend the traditional structure of clinical trials, and the consequences of poor recruitment and retention rates are there for all to see.
The evidence from decentralised trials underlines that people are far more willing to engage in these studies than current evidence suggests; pharma must demonstrate to patients that it’s capable of flexible and innovative practices, and acknowledge that it cannot continue with a one-size-fits-all approach to clinical trials.